
Helium is the 2nd element in the periodic table and has a symbol of He and atomic number of 2. It has an atomic weight of 4.00260 and a mass number of 4. Helium has two protons and two neutrons in its nucleus, and two electrons in one shell. It is located in group eighteen, period one and block s of the periodic table. Colourless, odourless gaseous nonmetallic element. Belongs to group 18 of the periodic table. Lowest boiling point of all elements and can only be solidified under pressure. Chemically inert, no known compounds. Discovered in the solar spectrum in 1868 by Lockyer.
In 2008, approximately 169 million standard cubic meters (SCM) of helium were extracted from natural gas or withdrawn from helium reserves, with approximately 78% from the United States, 10% from Algeria, and most of the remainder from Russia, Poland, and Qatar.[145] By 2013, increases in helium production in Qatar (under the company Qatargas managed by Air Liquide) had increased Qatar's fraction of world helium production to 25%, making it the second largest exporter after the United States.[146] An estimated 54 billion cubic feet (1.5×109 m3) deposit of helium was found in Tanzania in 2016.[147] A large-scale helium plant was opened in Ningxia, China in 2020.[148]
[source]
A helium mining operation is seen at the Nahata Dziil chapter of the Navajo Nation, near Chambers, Arizona, on April 8, 2021.
[source]
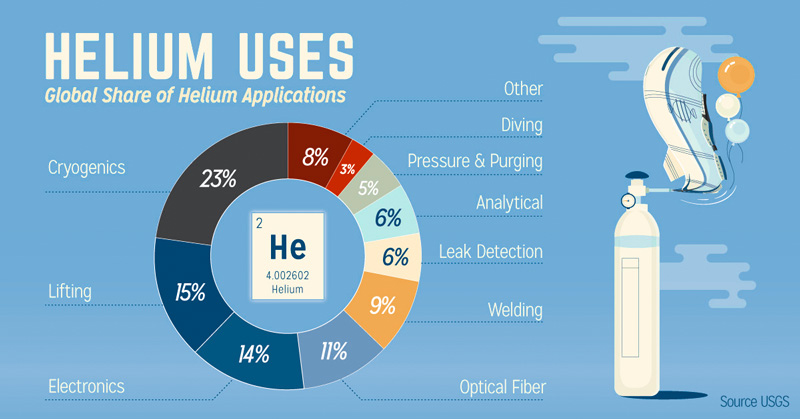
[source]

[source]
Medical uses of Helium
• Helium gas is crucial in medicine for various purposes, such as helping people with breathing difficulties and protecting the heart during reduced blood flow.
• Scientists are studying the potential benefits of helium in safeguarding the brain and improving patients' breathing during surgeries.
• Helium is also used in advanced medical technologies like MRI scans and organ imaging.
Helium gas plays a crucial role in the field of medicine. Its unique characteristics, such as being lightweight and having good heat conductivity, make it useful for various purposes. One important application is helping people with breathing difficulties like asthma and COPD. Helium can also protect the heart during periods of reduced blood flow. Scientists are still exploring how helium can safeguard the brain. Surgeons are even considering using helium instead of carbon dioxide in certain surgeries to improve patients' breathing. Additionally, helium is utilized in advanced medical technologies like MRI scans and detailed organ imaging.
[Source differentiated using Diffit]